Improved therapy
It is generally recognized that any use of antimicrobials selects for antimicrobial resistance but the relative contribution of different drugs, dosages and durations of treatment to antimicrobial resistance is poorly understood. We pursue the objective of improving therapy by understanding and reducing the contribution of each of these variables to development of antimicrobial resistance. This research line includes development of new tools for discovery of innovative antimicrobials, combination therapies based on reversal of antimicrobial resistance, pathogen-targeted therapies with minimal impact on the commensal microbiota, and alternatives to conventional antimicrobials.
These are the current projects in this area:
Alternatives to Veterinary ANTimicrobials (AVANT)Objective: to develop and test the efficacy and sustainability of alternatives to antimicrobials for the management of post-weaning diarrhoea in pigs. The alternatives under study include gut microbiome modulators based on synbiotic products or faecal microbiota transplantation, innovative medicines targeting the pathogen or stimulating the pig immune response, as well as feeding strategies for prevention of the disease. Based on the results of pre-clinical studies, including regulatory and implementation considerations, the efficacy of the three most promising interventions will be assessed by farm trials. Mathematical modelling will be used to quantify the benefits on reduction of antimicrobial use at the farm and EU level if these interventions were implemented in pig farming. |
European Network for Optimization of Veterinary Antimicrobial Treatment (ENOVAT)Objective: to optimize veterinary antimicrobial use with special emphasis on the development of animal- and disease-specific antimicrobial treatment guidelines and refinement of microbiological diagnostic procedures. This will be fulfilled through the contribution of five working groups, namely WG1 (Mapping microbiological diagnostics and treatment guidelines), WG2 (European strain database), WG3 (clinical breakpoints), WG4 (antimicrobial treatment guidelines) and WG5 (Dissemination). |
Re-sensitizing multidrug-resistant Escherichia coli to fosfomycin and macrolidesObjective: To identify a set of non-resistance genes/proteins that are essential for expression of macrolide and fosfomycin resistance in E. coli, and ultimately use them as drug targets to re-sensitize pathogenic E. coli to these antimicrobials. |
Identification of novel antibiotics by hypomorphic expression of essential genesObjective: to enhance discovery of truly novel antimicrobial classes that interfere with untapped bacterial proteins using a screening strategy based on CRISPRi (clustered regularly interspaced short palindromic repeats interference). |
Optimizing antimicrobial treatment length for cystitis and pyoderma in dogsObjective: to compare the clinical efficacy of two amoxicillin treatment regimens in dogs with cystitis, and two cephalexin treatment length in dogs with superficial pyoderma |
Assessment of cephalexin and amoxicillin clavulanate efficacy for canine MRSPObjective: to provide a safe and effective systemic option for MRSP infections that require systemic therapy by i) evaluating the clinical efficacy of cefalexin and amoxicillin clavulanate and ii) identifying MRSP isolates that display in vitro susceptibility to these β-lactam antibiotics. |
Potential novel antimicrobialsObjective: to investigate the potential of a collection of novel, synthetic compounds as antimicrobials. The investigations include both in vitro and in vivo experiments. Read more https://innovationsfonden.dk/da/investeringer/investeringshistorier (in Danish) |
Key references (last 5 years):
- Svanberg Frisinger F, Jana B, Donadio S, Guardabassi L. 2021. In silico prediction and prioritization of novel selective antimicrobial drug targets in Escherichia coli. Antibiotics. 10(6):632.
- Ronaghinia AA, Nikolaisen NK, Hansen SG, Poulsen HH, Frandsen HL, Struve T, Toutain PL, Damborg P. 2021. Validating an empiric sulfadiazine-trimethoprim dosage regimen for treatment of Escherichia coli and Staphylococcus delphini infections in mink (Neovison vison). J Vet Pharmacol Ther. 2021 Jan;44(1):93-106. doi: 10.1111/jvp.12894. Epub 2020 Sep 13.
- Ronaghinia AA, Birch JM, Frandsen HL, Toutain PL, Damborg P, Struve T. 2021. Evaluating a tylosin dosage regimen for treatment of Staphylococcus delphini infection in mink (Neovison vison): a pharmacokinetic-pharmacodynamic approach. Vet Res. 52(1):34.
- Wegener A, Damborg P, Guardabassi L, Moodley A, Mughini-Gras L, Duim B, Wagenaar JA, Broens EM. 2020. Specific staphylococcal cassette chromosome mec (SCCmec) types and clonal complexes are associated with low-level amoxicillin/clavulanic acid and cefalotin resistance in methicillin-resistant Staphylococcus pseudintermedius. J Antimicrob Chemother 75(3):508-511.
- Espinosa-Gongora C, Rem Jessen L, Nordang Kieler I, Damborg P, Reinhard Bjørnvad C, Dadi Gudeta D, Pires Dos Santos T, Sablier-Gallis F, Sayah-Jeanne S, Corbel T, Nevière A, Hugon P, Saint-Lu N, de Gunzburg J, Guardabassi L. 2020. Impact of oral amoxicillin and amoxicillin/clavulanic acid treatment on bacterial diversity and β-lactam resistance in the canine faecal microbiota. J Antimicrob Chemother75:351-361.
- Baker KR, Jana B, Hansen AM, Nielsen HM, Franzyk H, Guardabassi L. 2019. Repurposing azithromycin and rifampicin against Gram-negative pathogens by combination with peptidomimetics. Front Cell Infect Microbiol
- Baker KR, Jana B, Hansen AM, Vissing KJ, Nielsen HM, Franzyk H, Guardabassi L. 2019. Repurposing azithromycin and rifampicin against Gram-negative pathogens by combination with peptide potentiators.Int J Antimicrob Agents [Epub ahead of print].
- Greco I, Plahn Emborg A, Jana B, Molchanova N, Oddo A, Damborg P, Guardabassi L, Hansen PR. 2019. Characterization, mechanism of action and optimization of activity of a novel peptide-peptoid hybrid against bacterial pathogens involved in canine skin infections. Sci Rep9: 3679.
- Magnowska Z, Jana B, Brochmann RP, Hesketh A, Lametsch R, De Gobba C, Guardabassi L. 2019. Carprofen-induced depletion of proton motive force reverses TetK-mediated doxycycline resistance in methicillin-resistant Staphylococcus pseudintermedius. Sci Rep9:17834.
- Sadaka C, Damborg P, Watts JL. 2018. High-throughput screen identifying the Thiosemicarbazone NSC319726 Compound as a potent antimicrobial lead against resistant strains of Escherichia coli. Biomolecules 8:4.
- Adler DMT, Damborg P, Verwilghen DR. 2017. The antimicrobial activity of bupivacaine, lidocaine and mepivacaine against equine pathogens: An investigation of 40 bacterial isolates. Vet J 223: 27-31.
Key Research areas
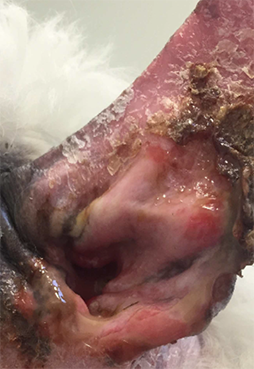
Dog ear infected by Pseudomonas aeruginosa


