Newborn Infection and Immunity
The goal of the Newborn Infection and Immunity group is to explore and understand the mechanisms of disease before and after birth – especially related to infections. We study how milk and microbial factors as well as immunometabolic drugs influence cell and organ development and resistance against infections. This is done using in vitro cell and ex vivo blood and tissue studies from animals and humans with and without infections. We explain why newborns are susceptiple to infections and how to improve their health status with interventions.
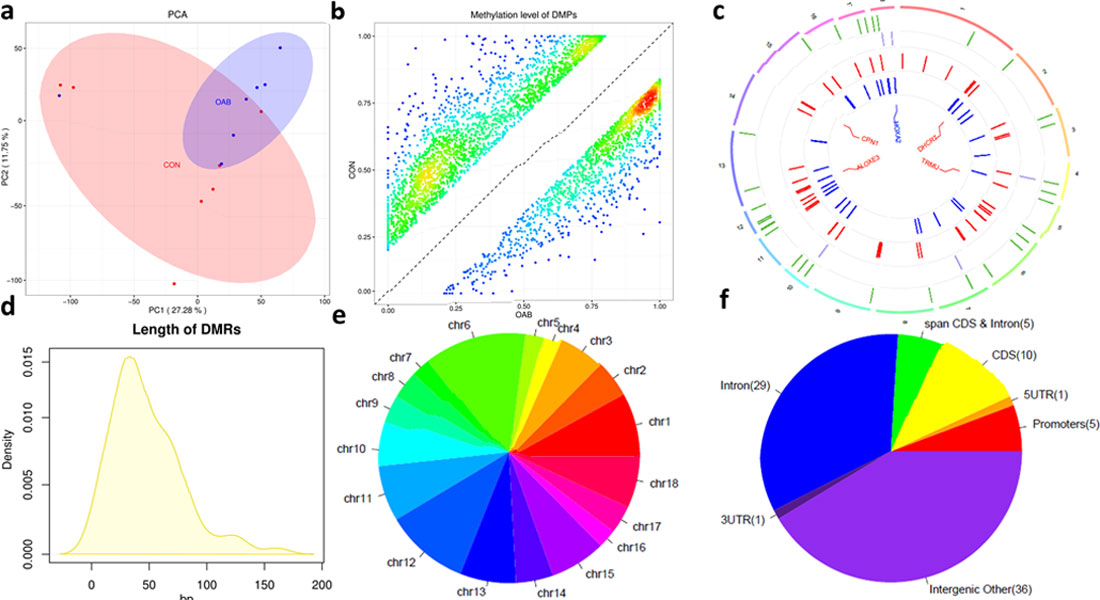
Newborn mammals are highly susceptible to infections, especially when born preterm or growth-restricted. Sepsis and necrotizing enterocolitis (NEC) are two important diseases. Optimal nutrition and gut colonization in early life affects health outcome, both short- and long-term.
Closely related to in vivo studies in animals or humans, we use ex vivo and in vitro intestinal, blood and brain cell models to show effects of milk components or drugs on epithelial integrity and cellular immunity. We use advanced -omics techniques (transcriptomics, proteomics, epigenomics) to characterize cells during development and in response to treatments. We also study the milk and microbial factors as well as drugs (antibiotics, immunometabolic drugs) that are protective or detrimental.
“Coupling information from cell models, animal studies and human trials has a great potential to provide a better mechanistic understanding of diseases in newborns. This leads to better diagnosis, prevention and treatment of important diseases around birth” says Associate Professor and group leader Duc Ninh Nguyen.”
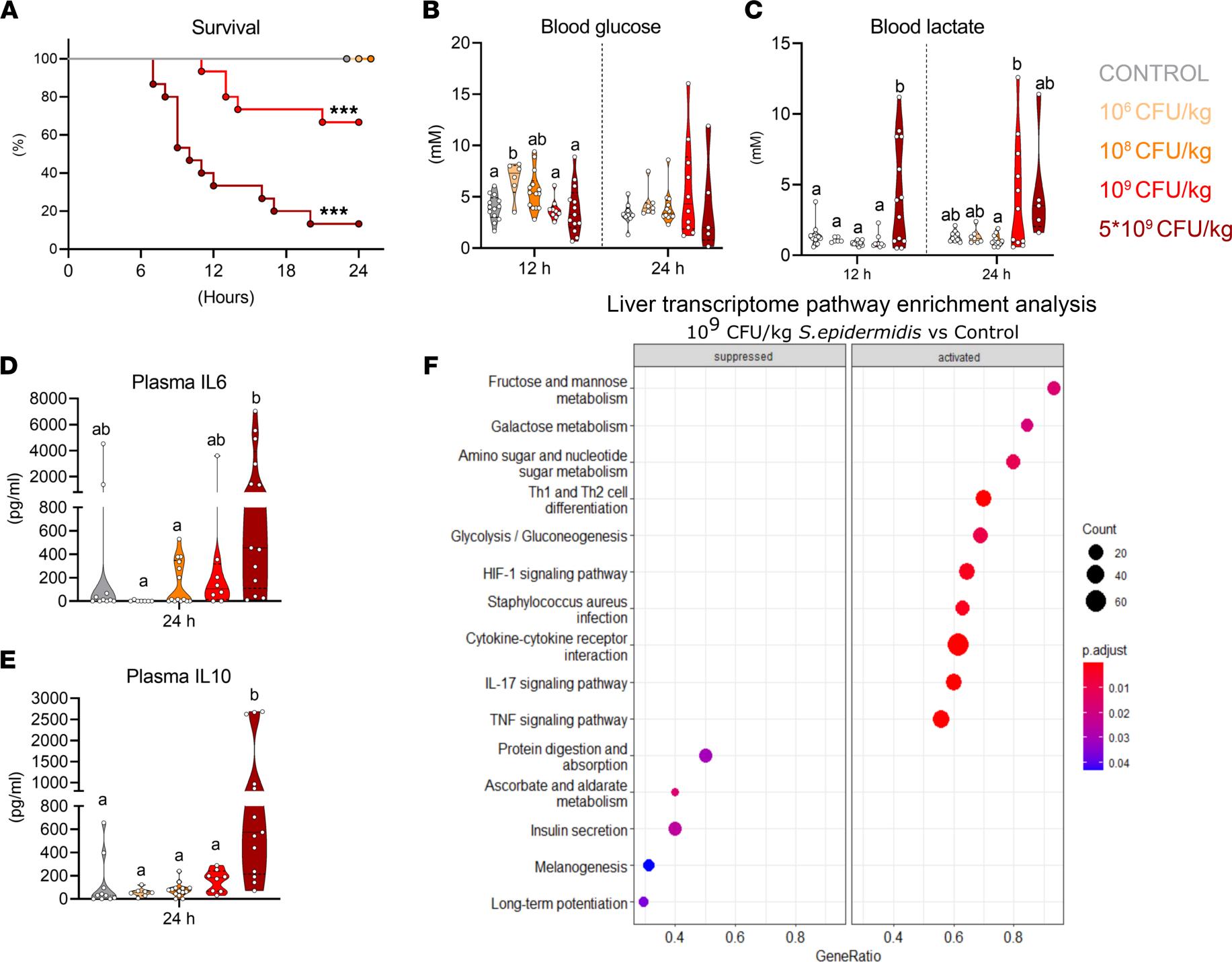
Due to organ immaturities, preterm infants cannot tolerate large amounts of enteral feeding, and they need further nutritional support with parenteral nutrition (PN) rich in glucose. Here, using a clinically-relevant animal model of neonatal sepsis, we showed for the first time that standard PN glucose use in neonatal clinics was highly detrimental for infected preterm newborns. The main reason was the high PN glucose supply accelerating systemic immune cell glycolysis, providing vast ATP amounts for inflammatory responses and causing organ dysfunctions. We also suggested interventions to maintain controlled glycolysis as well as a relatively low blood glucose levels, but not hypoglycemia, to prevent sepsis development in infected newborns. https://insight.jci.org/articles/view/157234
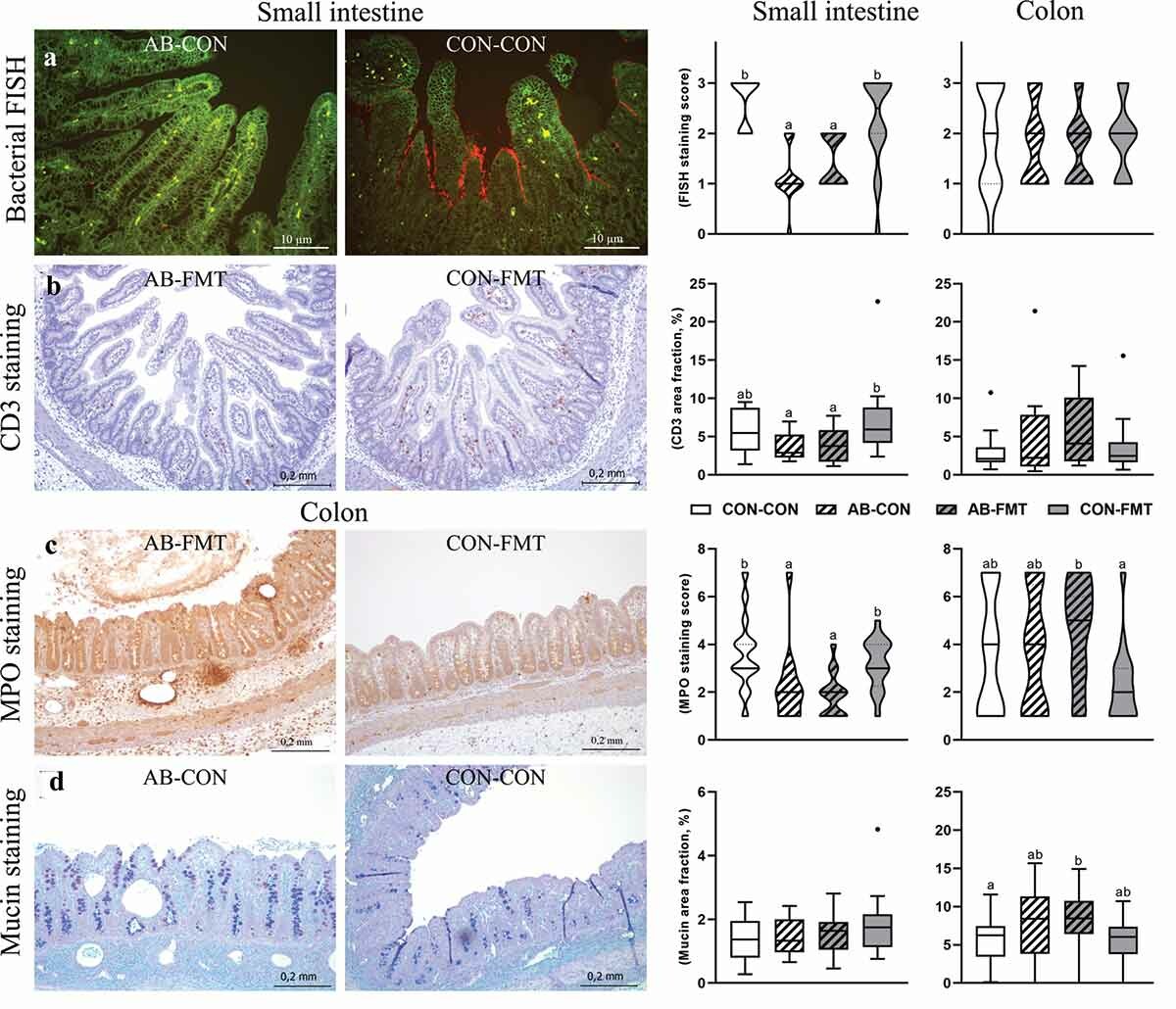
Fecal microbiota transplantation (FMT) has been shown to be promising to alleviate gut inflammation in many diseases, including necrotizing enterocolitis (NEC), via modulation of the gut microbiome. As most preterm newborns receive antibiotic treatment, here we hypothesized that FMT use right after antibiotic cessation can reverse any detrimental effects of antibiotics on gut dysbiosis and immune suppression. Testing this in the well-known NEC model in preterm piglets, we were surprised to observe that FMT antagonized the effects of antibiotics in the gut with worse NEC outcomes, and did not improve the systemic immune suppression induced by precedential antibiotic use. DOI: https://www.tandfonline.com/doi/full/10.1080/19490976.2020.1849997
1) Impacts of BCG vaccine on newborn immunity and neonatal sepsis risks: 2024-2027, funded by Novo Nordisk Foundation, 3.0 mio DKK
PI: Assoc. Prof. Duc Ninh Nguyen. Collaborators: Prof. Tobias Kollmann and Dr. Nelly Amenyogbe. In last few years, BCG vaccine has shown protective non-specific effects against neonatal infection and mortality in several African countries, which is paving the way for potential new WHO recommendation for BCG vaccination for newborns. To make this happen, we need more mechanistic insights. This project utilizes a preterm pig model to explore how BCG vaccine affects newborn immunity and neonatal sepsis risks.
2) Nutrition targeting systemic immune cell metabolism as therapy for neonatal infection: 2023-2026, funded by Novo Nordisk Foundation, 3.0 mio DKK.
PI: Assoc. Prof. Duc Ninh Nguyen. Collaborators: Profs Søren Skov and Tobias Kollmann, Assoc. Prof. M.D. Lise Aunsholt, postdoc Ole Bæk, PhD student Ziyuan Wu. Our main current interest is to explore immunometabolic modulators that can prevent neonatal death in infection-sensitive infants. Those modulators are nutrition, immunometabolic drugs and vaccines (via non-specific effects). This project includes series of studies in whole blood, infected preterm animals as well as a pilot trial in preterm infants to identify clinically-relevant approaches that can be tested in large randomized controlled trials in the future.
3) Human plasma-derived proteins to prevent neonatal sepsis and necrotizing enterocolitis: 2021-2024, funded by Takeda Pharmaceutical company, 6.2 mio DKK.
PI: Assoc. Prof. Duc Ninh Nguyen. Collaborators: Prof. Per Sangild, Postdoc Ole Bæk, PhD student Ziyuan Wu. In collaboration with Takeda, we identify potential human plasma-derived proteins with anti-inflammatory properties, and test for their NEC and sepsis-preventive effects using preterm pigs as animal models.
4) Phage the enemy-bacteriophages to fight necrotizing enterocolitis: 2021-2024, funded by Independent Research Fund Denmark (IRFD), 2.8 mio DKK.
PI: Assoc. prof. Duc Ninh Nguyen. Collaborators: Ass. Profs. Anders Brunse, Michela Gambino, Prof. Dennis Nielsen, PhD student Malene Spigelhauer. We have previously shown that fecal filtrate transplantation (containing bacteriophages) prevents NEC development in preterm pigs. In this project, we isolate NEC-associated bacteria from necrotic gut mucosa, and use fecal filtrates from multiple donors to identify phages that can eliminate NEC-associated bacteria via plaque assays. Then, a consortium of these phages are tested for NEC preventive effect in preterm pigs.
5) CASGUT- Caseins for gut comfort in infants: 2022-2025, funded by Arla Food for Health, 5.1 mio DKK.
PIs: Assoc. Prof. Stine Bering and Prof Lotte B. Larsen. Collaborators: Prof Per Sangild, Arla Foods Ingredients. The project explores potential benefit of gently-treated intact and hydrolysed micellar casein isolate as part of infant formula to improve gut comfort and motility.
6) NEOCOL- Colostrum for newborns, 2017-2024, funded by Innovation Fund Denmark, 18 mio DKK.
PI: Prof Per Sangild. Co-PIs: Assoc. Profs. Duc Ninh Nguyen and Stine Bering. Multiple international collaborators. We perform series of studies testing how bovine colostrum affect the newborn maturation of multiple organs and the immune system in both animals and preterm infants (3 RCTs). Additionally, we do various omics analyses of plasma and fecal samples from RCTs for cohort studies.
- Arla Food amba
- Arla Foods Ingredients
- Biofiber-Damino
- Innovation Fund Denmark
- University of Copenhagen
- Independent Research Fund Denmark (IRFD)
- Takeda Pharmaceutical Company
Currently, our research intensively focuses on:
- Impact of perinatal complications (preterm birth, pre-/post-natal infection and inflammation) on immune development in early life.
- Host, nutritional status and microbe interaction to determine health in early life.
- Immunometabolic mechanisms in neonatal infectious diseases (neonatal sepsis, necrotizing enterocolitis-NEC) and novel therapeutic approaches.
Our research platforms and tools are based on: 1) preterm pig model of neonatal sepsis and NEC; 2) observational studies in human preterm infant cohorts; 3) multi-omic analyses (transcriptomics, proteomics, gut microbiome); 4) External collaborations with Danish and international hospitals/research centers working with newborn infants, neonatal mice and preterm lambs.
We are continuously looking for MSc students to conduct MSc thesis research (30, 45 or 60 ECTS) in an ongoing long-term project to explore nutritional and pharmaceutical therapy for neonatal sepsis. The project utilizes preterm piglet model of neonatal sepsis to mimic conditions in infected preterm infants. Mechanistic insights are also explored using ex vivo blood immune assays as well as transcriptomic and metabolomic analyses. You will be involved in a research team including scientists with diverse backgrounds (medical doctors, immunologists, Vet scientists).
A previous background study using similar animal models and in vitro studies can be found here: https://doi.org/10.1172/jci.insight.157234
A detailed plan of the thesis can be adjusted based on the wishes and competences of the MSc students.
For more details, please contact Associate Professor Duc Ninh Nguyen (dnn@sund.ku.dk).
CONTACT
Dyrlægevej 68, building 1-73
1870 Frederiksberg C
Group/project members
| Name | Title | Phone |
|---|

